Bioequivalence: What It Means for Generic Drugs and Your Health
When you pick up a generic pill, you might wonder: bioequivalence, the scientific standard that proves a generic drug performs the same as its brand-name counterpart in the body. It’s not just a label—it’s the reason your $5 generic blood pressure pill works just like the $100 brand. Without bioequivalence, generics could be useless or even dangerous. But here’s the truth: the FDA doesn’t just approve generics because they look the same. They test them to make sure they release the same amount of medicine into your bloodstream at the same speed. That’s bioequivalence in action.
It’s not magic—it’s math. Scientists measure how much of the drug enters your blood (AUC) and how fast it gets there (Cmax). If the generic stays within 80% to 125% of the brand’s numbers, it’s approved. This isn’t a guess. It’s based on real studies with healthy volunteers. And it’s why a generic version of metformin or levothyroxine can replace the brand without you feeling any difference. But here’s what most people don’t know: bioequivalence doesn’t guarantee identical side effects. Some people react differently to fillers or coatings, even if the active ingredient is the same. That’s why switching between generic brands can sometimes cause issues—especially with narrow-therapeutic-index drugs like warfarin or thyroid meds.
Therapeutic equivalence, the clinical outcome that follows when bioequivalence is proven is what actually matters to you. If your blood pressure stays steady, your cholesterol drops, or your seizure frequency doesn’t change after switching to a generic, you’ve got therapeutic equivalence. But if you’ve ever felt worse after a refill change, you’re not imagining it. Some patients report subtle shifts—fatigue, dizziness, or mood changes—when switching between generic manufacturers. It’s rare, but real. That’s why doctors sometimes stick to one generic brand for sensitive conditions.
Drug absorption, how quickly and completely your body takes in a medication is the core of bioequivalence. A pill might have the same chemical structure, but if it dissolves too slow or too fast, your body won’t get the right dose. That’s why generic makers must prove their product behaves like the original under test conditions. The FDA doesn’t just check the label—they test the actual tablet in labs that mimic your stomach and intestines. And they require multiple batches to be consistent. That’s why you rarely see recalls of generics for absorption issues—it’s not a loophole, it’s a locked door.
What you’ll find below isn’t just a list of articles. It’s a practical guide to how bioequivalence affects real people. From parents worried about their child’s ADHD meds to seniors juggling five prescriptions, these stories show how generic drug policies, insurance rules, and even patient psychology shape what works—and what doesn’t. You’ll see how Medicaid cuts costs using bioequivalence standards, why some doctors still hesitate to prescribe generics, and how a second generic manufacturer can drop prices by 60%. This isn’t theory. It’s your medicine. And you deserve to know how it got there.
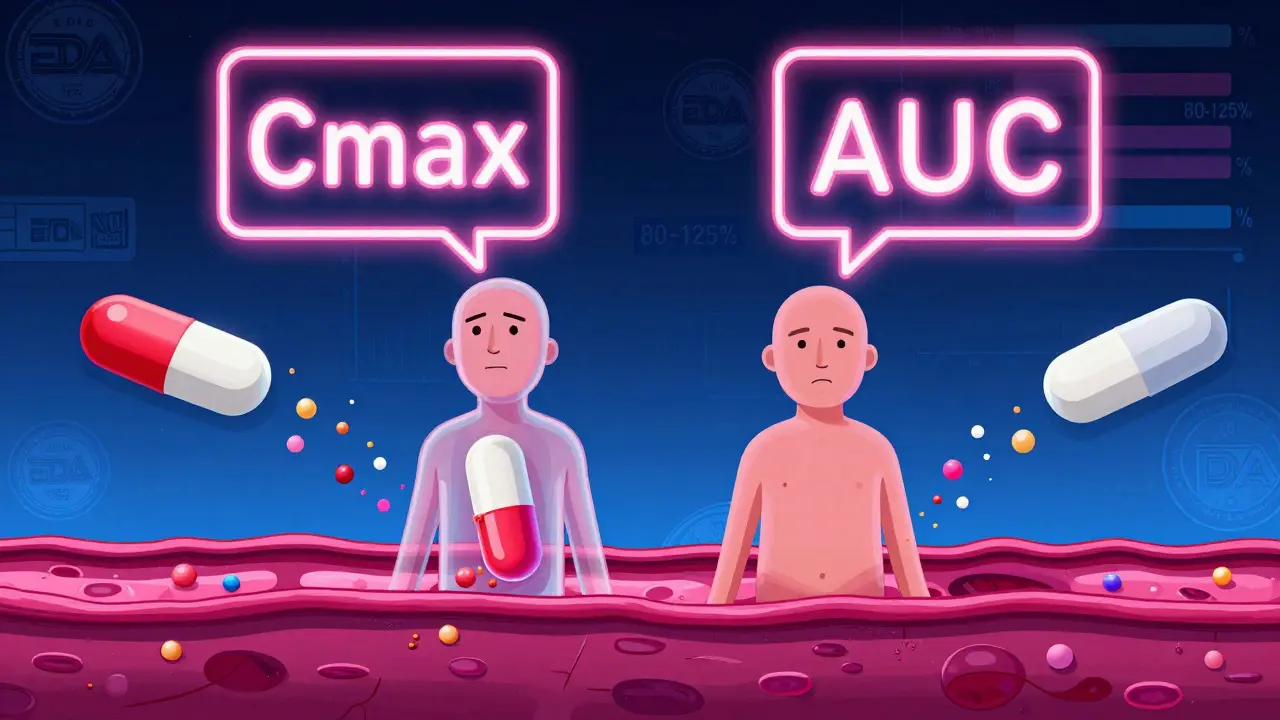
Bioequivalence Explained: FDA Requirements to Prove Generic Drug Equivalence
Learn how the FDA proves generic drugs work just like brand-name versions using bioequivalence studies, Cmax, AUC, and the 80-125% rule. Understand what really makes generics safe and effective.

Therapeutic Equivalence: What It Means for Patient Safety
Therapeutic equivalence ensures generic drugs work just like brand-name ones, saving money without risking safety. Learn how the FDA verifies this, why it matters for patients, and what to watch for when switching.
