The Hatch-Waxman Act didn’t just change how drugs get approved in the U.S.-it rewrote the rules of the entire pharmaceutical market. Before 1984, generic drugs were rare. Fewer than 10 got FDA approval each year. Today, 9 out of 10 prescriptions filled are for generics. That shift didn’t happen by accident. It was built on a carefully negotiated law that tried to do two things at once: protect drug innovators and let cheaper copies reach patients faster. The Drug Price Competition and Patent Term Restoration Act of 1984, better known as the Hatch-Waxman Act, is the reason you can buy a $4 blood pressure pill instead of a $400 one.
What the Hatch-Waxman Act Actually Did
The law created a legal shortcut for generic drug makers. Before it, any company wanting to sell a copy of a brand-name drug had to run the same expensive clinical trials as the original maker. That meant years of testing, millions in costs, and almost no chance of competing. Hatch-Waxman changed that by introducing the Abbreviated New Drug Application (ANDA). Generic companies no longer had to prove safety and effectiveness all over again. They just had to show their version worked the same way in the body as the original-what’s called bioequivalence. This cut development costs by about 75% and opened the door for hundreds of new generic drugs each year.
But letting generics in fast wasn’t the only goal. Brand-name drug companies were worried. Their patents were the lifeblood of their profits. If someone could copy their drug the moment the patent expired, they’d lose their return on investment. So Hatch-Waxman gave them something back: patent term restoration. If a drug took five years to get FDA approval, the company could get up to five years added to its patent clock. The average extension? Just over two and a half years. That gave innovators more time to recoup costs before generics showed up.
The Safe Harbor That Changed Everything
One of the most powerful parts of the law was a little-known clause: 35 U.S.C. §271(e)(1). It created a legal safe harbor. Before Hatch-Waxman, even testing a patented drug before the patent expired was considered infringement. That meant generic makers couldn’t start preparing until the patent expired. The law flipped that. It allowed generic companies to begin making, testing, and studying patented drugs during the patent term-as long as the only goal was to get FDA approval. This meant generics could line up their applications years in advance. The result? The moment a patent expired, generics were ready to hit the market. No waiting. No delays. Just competition.
How Generics Fight for Their First Shot
The law didn’t just make it easier to enter the market-it gave the first generic company to file a big reward. If a generic applicant claimed that a patent listed in the FDA’s Orange Book was invalid or wouldn’t be infringed (called a Paragraph IV certification), they could get 180 days of exclusive rights to sell their version before any other generic could. This created a rush. Companies would submit applications the moment they could, sometimes camping outside FDA offices just to be first. In 2003, the FDA changed the rules to let multiple companies share the exclusivity if they filed on the same day. But the incentive stayed. The first filer still had a huge advantage, especially for blockbuster drugs.
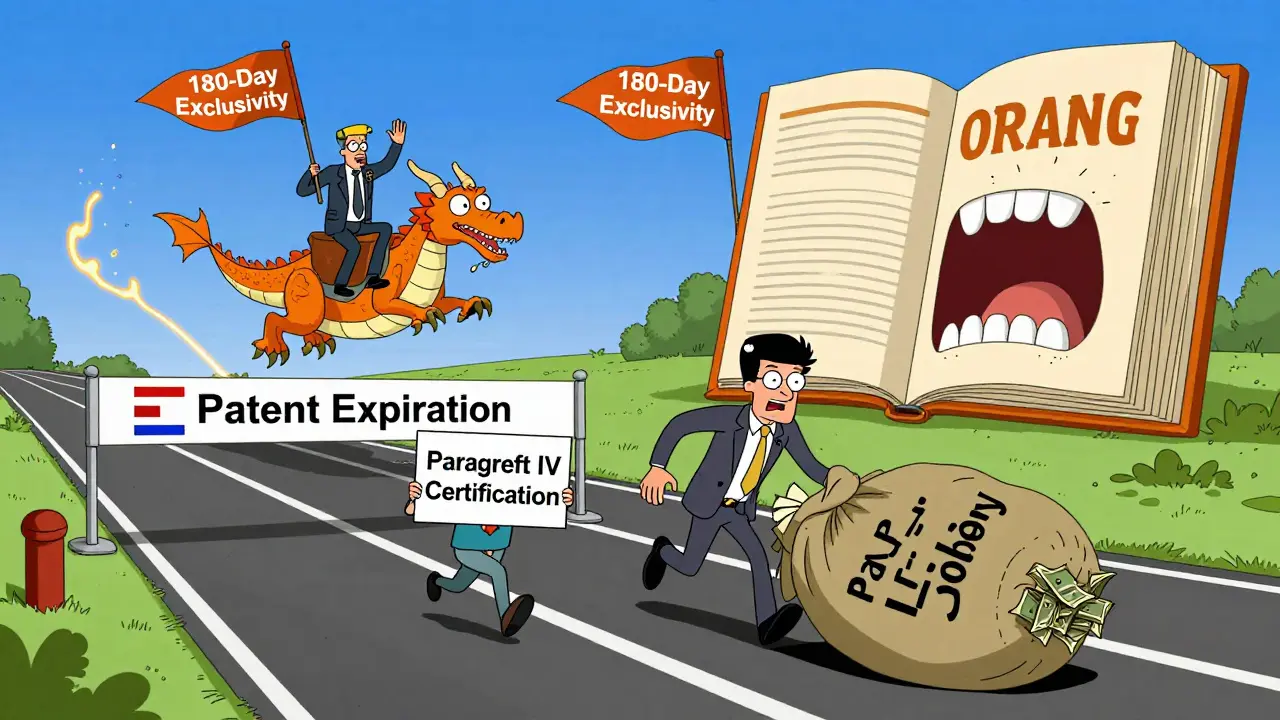
The Dark Side: Patent Games and Delay Tactics
For every win, there was a loophole. Brand-name companies didn’t sit still. They started filing more patents-not just on the drug itself, but on tiny changes: a new pill coating, a different dosage form, a slightly altered use. These are called secondary patents. In 1984, the average drug had about 3.5 patents. By 2016, that number jumped to 2.7 per drug. Today, it’s common for a single drug to have 14 or more patents. This creates a patent thicket-a maze of overlapping protections that makes it hard for generics to challenge even one without getting tangled in dozens of lawsuits.
Another tactic? Product hopping. A brand company slightly changes its drug-say, switching from a pill to a capsule-and then pushes doctors and patients to use the new version. The old one gets pulled from the market. Suddenly, generics can’t copy the old version anymore. The new version gets a fresh patent clock. Patients get stuck paying more, and generics get pushed back.
Then there’s pay-for-delay. In some cases, brand companies pay generic makers to hold off on launching their cheaper version. Between 2005 and 2012, about 10% of all generic patent challenges ended this way. The FTC called it anti-competitive. Congress tried to ban it with the Preserve Access to Affordable Generics Act in 2023, but enforcement remains weak.
The Numbers Don’t Lie
The impact is staggering. Since 1984, the U.S. has saved over $1.18 trillion on drug costs thanks to generics. In 2022 alone, generic drugs saved the system $313 billion. They make up 90% of prescriptions but only 18% of total spending. That’s the power of competition. But here’s the twist: while generics are cheap, getting them approved is harder than ever. An ANDA submission now averages 30,000 to 50,000 pages of paperwork. Legal battles over patents can cost $15 million to $30 million per case. The FDA’s review time dropped from 36 months to 10 months thanks to user fees, but 43% of applications still get rejected on first review for technical flaws.

Who Wins? Who Loses?
Patients win when generics enter the market. Prices drop to 15% of the brand-name price within six months. But the system is rigged in ways most people don’t see. Small generic companies struggle to afford the legal battles. Big ones-like Teva, Mylan, and Amneal-now control 62% of the market, up from 38% in 2000. Meanwhile, brand companies are getting more protection than ever. The average effective market exclusivity for a new drug is now 13.2 years, up from 10.4 years in 1984. That’s because of patent extensions, litigation delays, and ever-growing patent thickets.
The FDA has started pushing back. In 2022, it issued draft guidance to crack down on improper patent listings in the Orange Book. In 2023, it announced plans to cut ANDA review times to 8 months by 2025. The CREATES Act of 2022 stopped brand companies from blocking generic makers from getting drug samples needed for testing. But these are fixes, not overhauls.
Is the System Still Working?
Yes-but barely. The core idea of Hatch-Waxman still works: incentivize innovation while enabling competition. Without it, the U.S. would have seen 30-40% fewer new drugs approved between 1984 and 2018. But the balance has tilted. Patent abuse is now a $149 billion problem every year. The system was designed for a simpler time. Today, it’s a battlefield where legal teams outnumber scientists.
The future of generic drugs depends on whether regulators can tighten the loopholes without breaking the incentives. If they go too far, innovation could slow. If they do nothing, prices will keep climbing. The Hatch-Waxman Act was never meant to be permanent. It was meant to be a bridge. And right now, that bridge is showing cracks.
What is the ANDA pathway under the Hatch-Waxman Act?
The Abbreviated New Drug Application (ANDA) is the streamlined approval process created by the Hatch-Waxman Act for generic drugs. Instead of running full clinical trials, generic manufacturers only need to prove their product is bioequivalent to the brand-name drug-meaning it delivers the same amount of active ingredient into the bloodstream at the same rate. This cuts development time and cost by about 75%, making generic drugs affordable and widely available.
How does patent term restoration work?
Patent term restoration allows brand-name drug companies to extend their patent life to make up for time lost during FDA review. The law lets them add up to five years to their patent, but the actual average extension is 2.6 years. This helps recoup R&D costs before generics enter the market. The USPTO reviews each request and only grants restoration for time spent in regulatory review-not for delays caused by the company.
What is a Paragraph IV certification?
A Paragraph IV certification is when a generic drug applicant claims that a patent listed in the FDA’s Orange Book is either invalid or won’t be infringed by their product. This triggers a 45-day window for the brand-name company to sue for patent infringement. If they do, FDA approval is automatically delayed for 30 months. This is the main legal tool generics use to challenge patents early and get to market faster.
Why do generic drug approvals take so long now?
Even though the FDA now reviews ANDAs in about 10 months on average, the process is slower because applications are more complex. Generic companies must navigate dozens of patents, submit tens of thousands of pages of data, and often face legal challenges that delay approval. Many applications are rejected on first review due to technical errors, requiring resubmission. The legal and regulatory burden has grown far beyond what the original law envisioned.
What’s the difference between Hatch-Waxman and the EU’s drug approval system?
The EU uses a centralized approval system that started in 1995, which allows generics to enter the market faster after patent expiration-about 1.8 years sooner than in the U.S. But the U.S. system has more patent litigation: an average of 34 challenges per drug versus 12 in Europe. The U.S. model encourages more competition through legal challenges, while the EU prioritizes efficiency and fewer lawsuits. Neither system is perfect, but the U.S. model has delivered more generic options overall.
What Comes Next?
The Hatch-Waxman Act is 40 years old. The pharmaceutical world has changed dramatically since then. Biologics, biosimilars, and complex drug delivery systems weren’t even on the radar in 1984. The law is being stretched thin. Reform is coming-not to scrap it, but to fix its flaws. The focus now is on stopping pay-for-delay deals, limiting patent thickets, and making the Orange Book more transparent. If these changes stick, generics could enter the market 1.4 years faster by 2030, saving an extra $45 billion a year. But if they fail, the gap between brand and generic prices will widen again. The balance Hatch-Waxman created was fragile. It’s up to regulators, lawmakers, and the public to keep it from tipping over.


Comments
Chloe Hadland
January 26, 2026 AT 10:33Wow this is actually one of those laws that changed everyday life without most people even noticing. I never thought about why my blood pressure med is $4 instead of $400. Thanks for laying it out like this.
Vatsal Patel
January 27, 2026 AT 17:28So let me get this straight - we invented a system where the only way to innovate is to game patents like a board game and then pay off your competition to stay rich? Genius. Truly. The American Dream™ in a pill bottle.
Alexandra Enns
January 28, 2026 AT 07:43Canada doesn’t have this mess. We don’t let pharma companies stretch patents like taffy. We don’t let them pay generics to sit on their hands. We don’t have 14 patents on a damn aspirin. And yet somehow we still get new drugs. Funny how that works. Maybe we should export our system instead of importing your corporate circus.
John McGuirk
January 28, 2026 AT 17:32They're all lying. The FDA is controlled by Big Pharma. The 'safe harbor' clause? Just a front. They're all in on it. You think the 180-day exclusivity is for competition? Nah. It's a bribe. The same people who wrote the law own the patents. Watch the news - they're already moving to privatize the FDA next year. I saw it on a forum.
Darren Links
January 29, 2026 AT 08:26Honestly this is one of the few times government and industry actually found a middle ground. Yeah it’s messy now but without Hatch-Waxman we’d still be waiting 10 years for generics and prices would be insane. The system’s broken but the idea was brilliant.
Shanta Blank
January 30, 2026 AT 06:35Let me just say this - if you’re not outraged by pay-for-delay deals, you’re either on the payroll or you’ve been brainwashed by pharma ads during your morning coffee. This isn’t capitalism. This is feudalism with better packaging.
Sharon Biggins
January 31, 2026 AT 15:38i just want to say thank you for writing this it made me feel less alone in wondering why my meds cost so much even though theyre old. im so glad there are people trying to fix this
Sawyer Vitela
January 31, 2026 AT 15:48ANDA submissions are 50k pages? That’s not regulation. That’s torture. Someone’s getting paid to write all that. Probably a lawyer.
Kat Peterson
February 1, 2026 AT 22:52So basically the system was designed by lawyers who thought they were geniuses… and now we’re all paying for it with our health? 😭💔 I’m crying. I’m so emotionally exhausted.
Kevin Waters
February 2, 2026 AT 11:34Actually the 180-day exclusivity was meant to incentivize the hard work of challenging patents. The problem isn’t the rule - it’s how it’s exploited. Fix the loopholes, don’t throw out the whole system. We need more people willing to do the legal heavy lifting, not less.
Michael Camilleri
February 3, 2026 AT 11:16People don't realize that without patent extensions we'd have no new drugs at all. You want cheap meds? Fine. But then stop complaining when the next cancer cure never gets made because no one can afford to risk billions on research
Himanshu Singh
February 3, 2026 AT 11:22Life is a balance between profit and purpose. Hatch-Waxman tried to hold that line. Now we’ve tipped too far toward profit. The question isn’t whether to change it - it’s whether we have the courage to change it before it breaks us.
lorraine england
February 5, 2026 AT 04:05the fact that we’ve saved over a trillion dollars is insane. imagine if that money went to schools or roads instead. but hey at least we got our $4 pills right? 😅
Marie-Pier D.
February 5, 2026 AT 19:47Thank you for sharing this 🙏 I’m so grateful to know how this works now. I’ve been on generics for years and never realized how much effort went into making them affordable. You’ve given me a new appreciation for the system - even with its flaws. Let’s keep pushing for change, gently but firmly 💛
Tiffany Wagner
February 6, 2026 AT 22:08so wait the first generic company gets 180 days to make money off a drug then everyone else can jump in? that seems kinda unfair but also kinda smart? idk i just buy the cheap ones